News > Fact Sheets
How COVID-19 Vaccines Are Made
Posted on Dec 22 2020
12/21/2020
The first COVID-19 vaccines have come out within a year of the COVID-19 virus being discovered. There are many questions about how a vaccine could be created so quickly. With help from the federal government, the process was able to happen faster and more efficiently. It is important to know that steps to check for safety were not skipped. These timelines show how the process to make COVID-19 vaccines was made more efficient compared to how other vaccines are made.
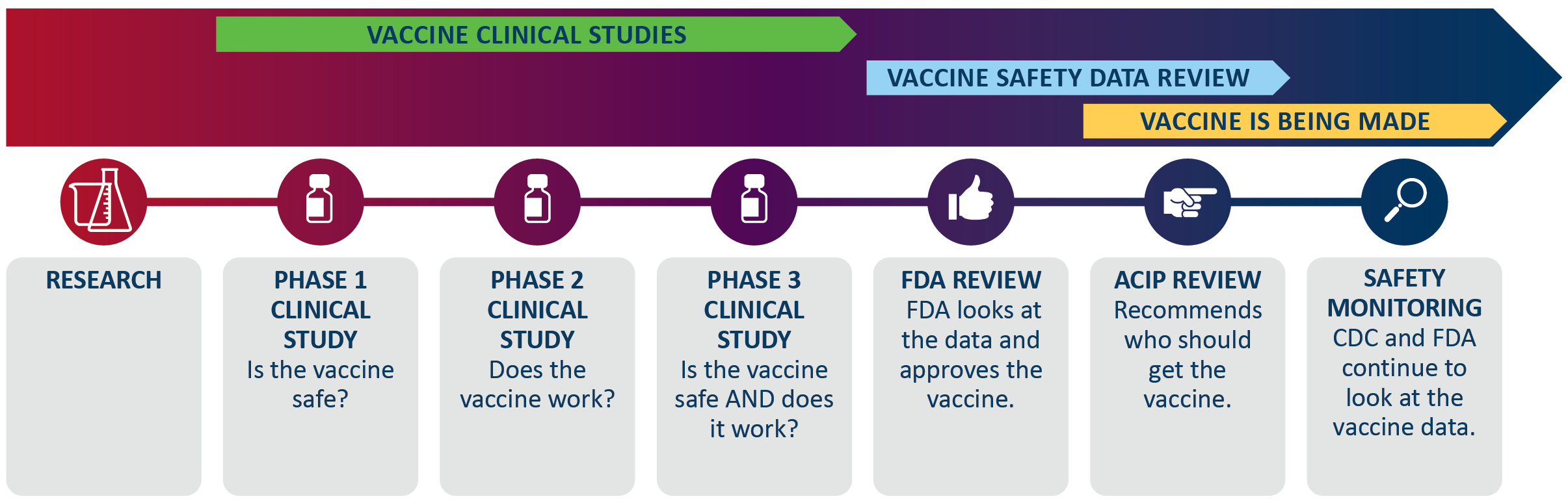
How vaccines are made
The process to make a vaccine can take up to 10 years.
- After research is done, a vaccine goes through three human clinical studies; each one has more and more people.
- The Food and Drug Administration (FDA) approves the vaccine after their science advisory group reviews the study results.
- Then a national advisory group, the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention (CDC), recommends who should get the vaccine.
- After that, the manufacturer starts to make vaccine.
- As people get the vaccine, CDC and FDA continue to look at the safety data for the vaccine.
A vaccine process starting with research. Then vaccine clinical studies. Phase 1 clinical studies happen first where they find if the vaccine is safe. Then phase 2 clinical studies where they find if the vaccine works. And then phase 3 clinical studies where they find if the vaccine is safe and if it works. Next, the vaccine safety data review. The FDA review is when FDA looks at the data and approves the vaccine. Then the ACIP review to recommend who should get the vaccine. Safety monitoring is last, where CDC and FDA continue to look at the vaccine data. Vaccine starts being made after the FDA review and continues past safety monitoring.
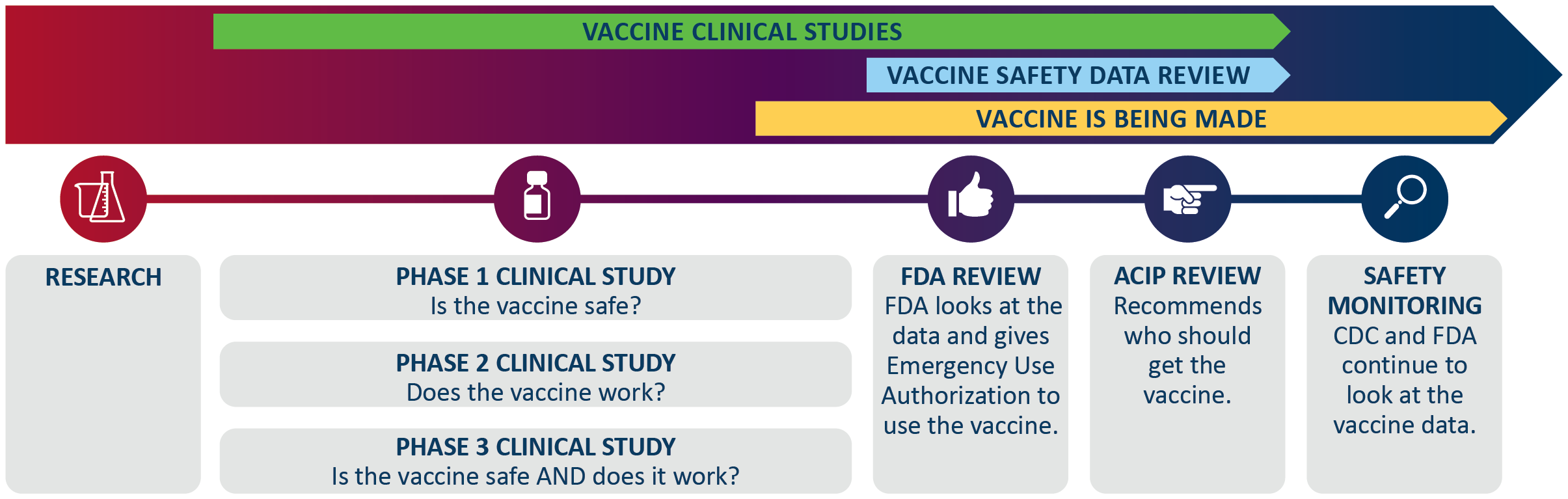
How COVID-19 vaccine is being made
- Earlier research on the coronavirus and advances in vaccine technology gave this process a jump start.
- Sometimes finding money for the vaccine studies can take a lot of time, but for COVID-19 vaccine, the federal government provided a lot of money.
- Manufacturers recruited participants for all three phases of the clinical studies at the same time, instead of waiting for each one to be done.
- Manufacturers are also making vaccine while the clinical studies happen.
- Approving a vaccine with an Emergency Use Authorization (EUA) also takes less time. Getting a safe vaccine that works is the number one priority.
- Groups at the federal level are not letting anything delay them from reviewing and hopefully approving vaccines. For example, the FDA added a lot of staff to shorten the review process from months to weeks.
- The vaccine will only receive approval if the studies show that the vaccine is safe and works.
A vaccine process starting with research. Then vaccine clinical studies. All three phases are happening at the same time. Phase 1 clinical studies happen first where they find if the vaccine is safe. Then phase 2 clinical studies where they find if the vaccine works. And then phase 3 clinical studies where they find if the vaccine is safe and if it works. The vaccine clinical studies go until the end of the ACIP review. Vaccine starts being made near the end of the phase 1, 2, and 3 clinical studies and extends past safety and monitoring. Next, the vaccine safety data review. The FDA review is when FDA looks at the data and gives emergency use authorization to use the vaccine. Then the ACIP review to recommend who should get the vaccine. Safety monitoring is last, where CDC and FDA continue to look at the vaccine data.
There are still things that we need to learn about the COVID-19 vaccine, such as how long protection from the vaccine lasts and how well it will work in the general population, but these are not reasons to delay getting a safe and effective COVID-19 vaccine out to people.
For more information, see COVID-19 Vaccine (www.health.state.mn.us/diseases/coronavirus/vaccine.html).
Minnesota Department of Health | health.mn.gov | 651-201-5000
625 Robert Street North PO Box 64975, St. Paul, MN 55164-0975
Contact health.communications@state.mn.us to request an alternate format.